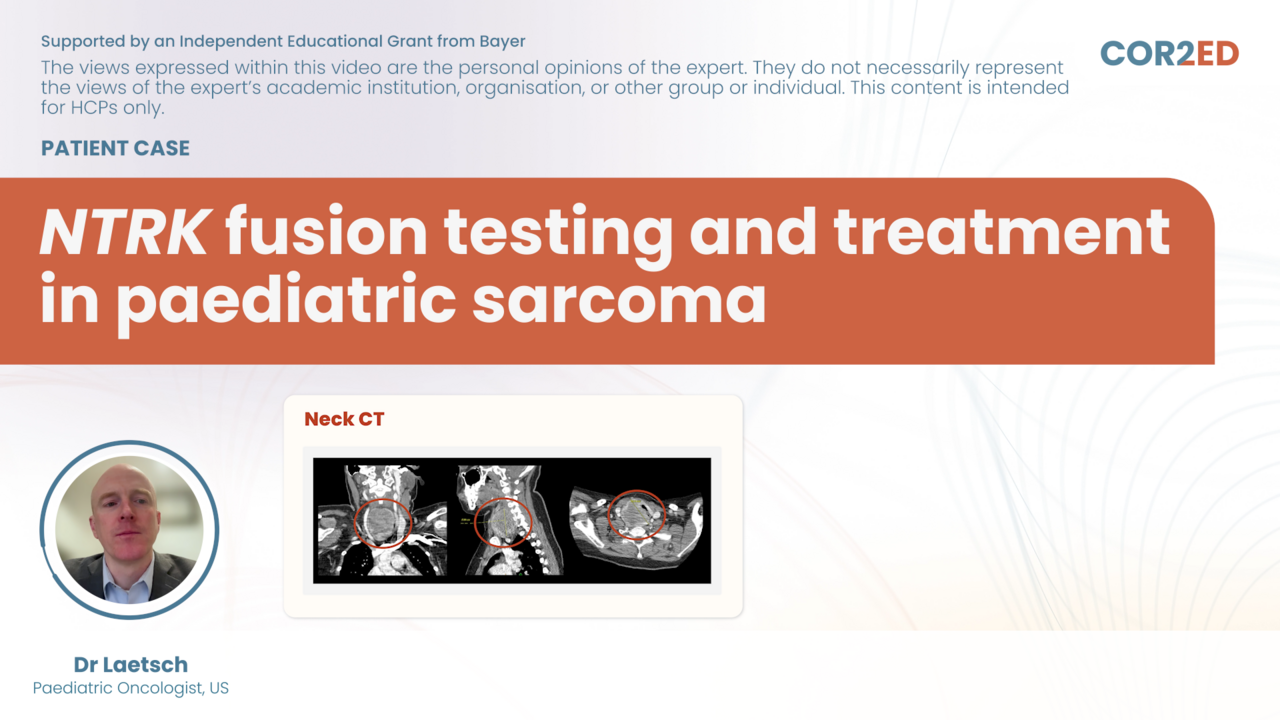
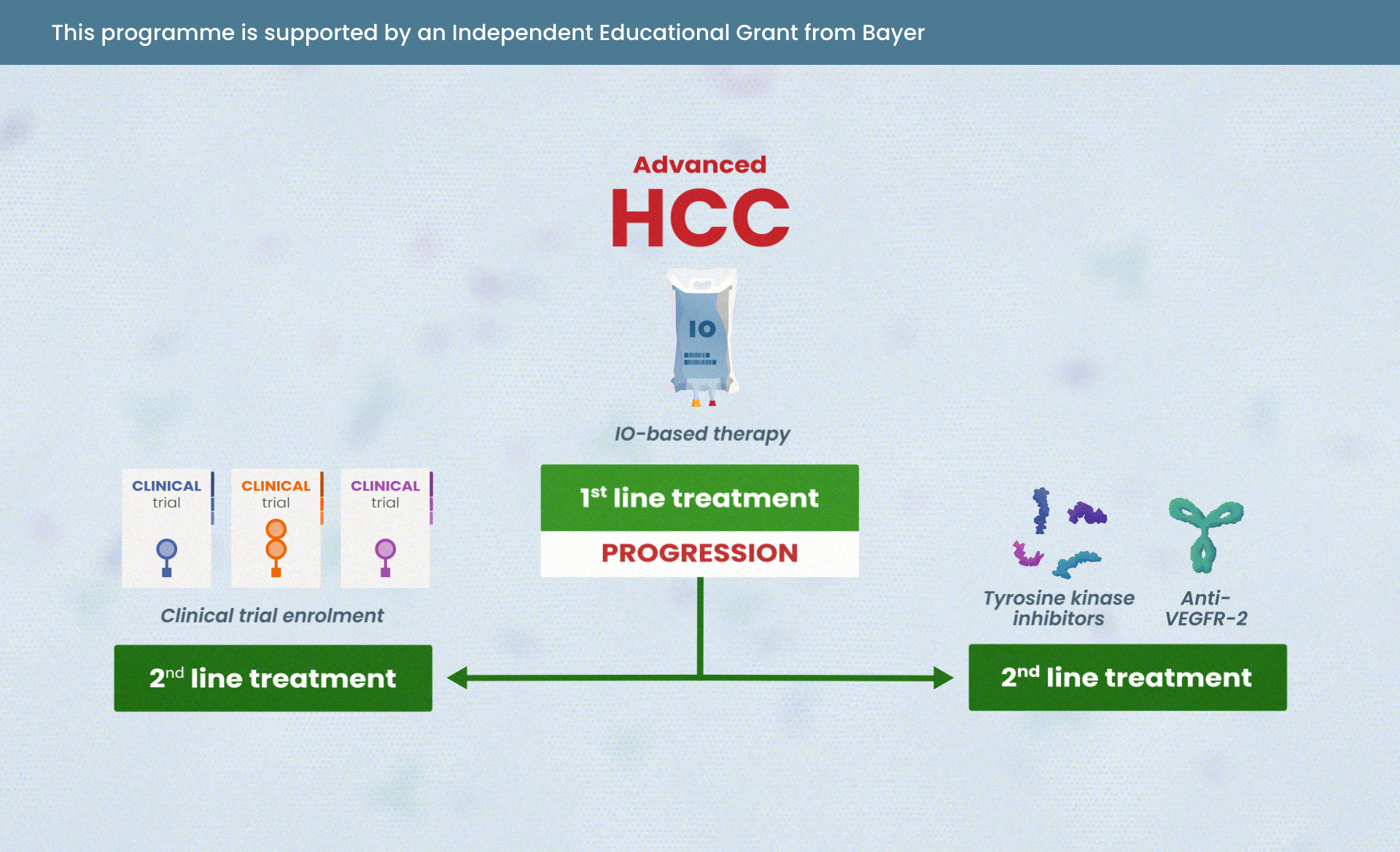
Medical experts come together to share their views in this on-demand webinar replay of an interactive online discussion:
New Oral Endocrine Therapy Options for Patients with Advanced or Metastatic ER+ Breast Cancer
-
The independent expert panel discussed oral SERDs efficacy and safety profiles in patients with ER+/HER2- advanced or metastatic breast cancer and their place in the treatment landscape
-
They also covered how to optimise treatment selection and sequencing in ER+/HER2- advanced or metastatic breast cancer and why, when and how to test for ESR1 mutation
-
The event concluded with speakers and participants engaging in a Q&A to address pressing questions
Watch the replay, broken down into 4 digestible videos, download the accompanying slides, and take the accredited e-learning using the blue button below the videos.
Clinical Takeaways
-
Elacestrant is the 1st oral SERD to be FDA approved (January 2023) for postmenopausal women or adult men with ER+/HER2-, ESR1-mutated advanced or metastatic breast cancer
-
-
FDA approved Guardant360 CDx assay as a companion diagnostic device to identify patients with ER+/HER2- advanced or metastatic breast cancer for treatment with elacestrant
-
-
With the aim of redefining treatment landscapes for ER+/HER2- advanced and metastatic breast cancer, several oral SERDs are in clinical development both as monotherapy and in combination therapy with other targeted therapies, including CDK4/6i
-
ESR1, PI3K, CDK4/6, BRCA, and AKT pathways alterations can be used as targets to guide treatment selection and sequencing decisions in ER+/HER2- advanced or metastatic breast cancer
-
ESR1 mutational status can be determined without repeated tissue biopsy of a metastatic site; instead, it can be done reliably by liquid biopsy at recurrence or progression on ET













 Downloadable
Downloadable  5 MIN
5 MIN
 Jun 2025
Jun 2025