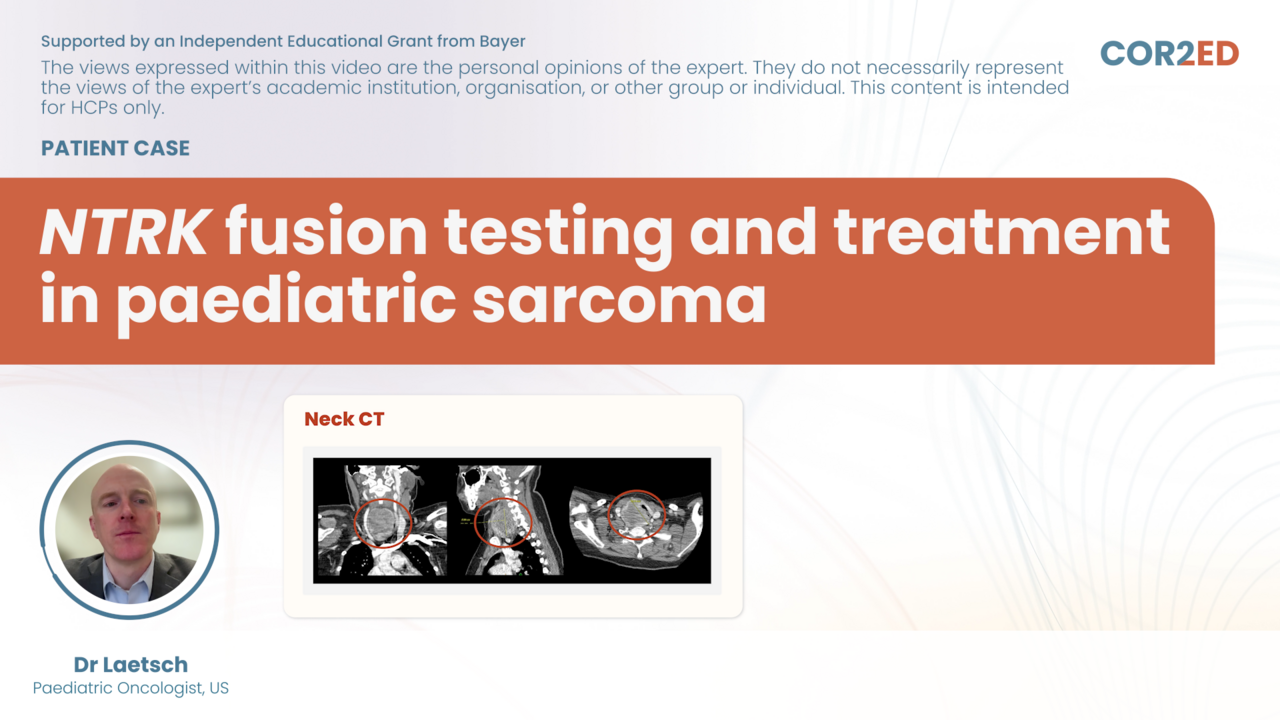
Podcast: Flexible Dosing of Oral Treatments in mCRC
Please note:
GI NURSES CONNECT podcasts are designed to be heard. If you are able, we encourage you to listen to the audio, which includes emotion and emphasis that is not so easily understood from the words on the page. Transcripts are edited for readability. Please check the corresponding audio before quoting in print.
This GI NURSES CONNECT programme is supported through an independent educational grant from Bayer and is an initiative of COR2ED.
The views expressed within this podcast are the personal opinions of the authors. They do not necessarily represent the views of the author’s academic institution, or the rest of the GI CONNECT and GI NURSES CONNECT groups.
Transcript
Sven De Keersmaecker
Hello, and welcome to this podcast on flexible dosing of oral treatments in metastatic colorectal cancer. I’m Sven De Keersmaecker and I'm a registered nurse and work as a study coordinator at Antwerp University Hospital in Belgium, and I'm also a member of GI NURSES CONNECT. I’m delighted to be joined today by Dr Gerald Prager who is a medical oncologist and Associate Professor at Medical University of Vienna and also a member of our physician partner group GI CONNECT. Thanks for joining me today Gerald.
Gerald Prager
Thank you, Sven. It's my pleasure to be here.
Sven De Keersmaecker
So Gerald, we are focusing our discussion today on flexible dosing of oral treatments in metastatic colorectal cancer, perhaps you can start off by taking us through the oral treatment options for these patients and the advantages of these oral therapies.
Gerald Prager
So there are several advantages when it comes to oral formulations of active drugs compared to IV treatment, especially in the time of the pandemic. Oral drugs, of course, can be taken at home by the patients, so they do not have to come in on a regular basis to the day clinic to get their chemotherapy treatment. It is also convenient for the patients as they are more flexible for travelling or whatsoever daily life activities. And this is important.
So let me first start with capecitabine. Capecitabine is an oral formulation of fluoropyrimidine, and we have it in use since more than 15 years now in the treatment for metastatic colorectal cancer. Capecitabine is a standard use in the adjuvant setting, especially in Stage Two colorectal cancer, but it's also used in the metastatic setting most likely in combination. Either in first line with bevacizumab for elderly or frail patients, or as a maintenance treatment.
So, when we de-escalate more aggressive chemo combinations in first line and then eventually step down the aggressiveness, we use capecitabine in combination with an antibody. We have learned to handle adverse events of capecitabine which are most likely GI toxicity like diarrhoea, stomatitis, but this drug might also cause hand-foot syndrome. Which is sometimes, if it's high grade, a hurdle for patients. And so we have learned to react on this, so proactively by hand creams, but also reactively by stepping down with the dosage when it's necessary.
When we go to later line of treatment, we have two oral drugs available after failure of IV treatment options, one of these drugs is regorafenib. Regorafenib is a totally different drug because regorafenib is a multi kinase inhibitor. So, this means we are interacting not only with the metabolism of the cancer cells, but also with the tumour micro-environment. Regorafenib is interacting with the immune response; it's interacting with angiogenesis by blocking the angiogenesis of the blood vessels that supply tumour cells, but it also interacting with the tumour cell biology itself.
Regorafenib is normally taken by three weeks on one week off regimen, so the patients are taking this drug three weeks in a row, and then they have one week off, they have a treatment break. So the patients are taking this medication as immunotherapy at home. Regorafenib can also cause adverse events such as fatigue, GI toxicity, hand-foot skin reactions or other skin reactions. We have learnt how to handle this drug quite well. That's important to do informed consent with our patients before they start this treatment.
The secondary oral drug in later line of treatment is trifluridine/tipiracil. Trifluridine/tipiracil, as the name is already suggesting, is the drug combination of two different drugs, which is essentially leading to the active compound, trifluridine to have a longer half-life time. Trifluridine/tipiracil is also an anti-metabolic drug. It's a member of the fluoropyrimidines and the patients are taking this drug in, let's say, quite a sophisticated manner because they’re taking five days the drug, two days off, five days the drug and 16 days off. So, it's important to educate the patients for the right drug scale intake and adverse events of these drugs are more haematotoxic adverse events. We have to monitor the blood cell count. We have especially to monitor the white blood cell count because low white blood cell count, as you know, might impede the immune response. Especially in the time of the pandemic, it is important that our patients are not on an increased risk for infections. And so the patients, although it's an oral drug, have to do blood cell monitoring on a regular basis.
Sven De Keersmaecker
Thanks for that, Gerald. So you have just mentioned some of the side effects associated with these treatments. We need to balance extended survival with the patient’s quality of life. So it's important for us to convey potential side effects to our patients and ensure they understand the strategies we use to manage this. Could you please take us through some of the differences in side effects associated with these treatments?
Gerald Prager
Yes, sure Sven. So let me first start with regorafenib. Regorafenib is a totally different drug than we are used to have from chemotherapy. It's a multi kinase inhibitor and we know it from other diseases that multi kinase inhibitors have different adverse events when compared to chemotherapeutic agents. So let me first start with all the drugs which are targeting angiogenesis. They have an adverse event potential risk of an increase in blood pressure. So we have to make sure that our patients are monitoring the blood pressure and eventually taking action by taking antihypertensive drugs.
A second adverse event which regorafenib might cause is GI toxicity. So for instance, diarrhoea. So, this is not an infectious diarrhoea. So, we can handle it by prescribing loperamide to our patients so that they can handle the diarrhoea. Other adverse events of kinase inhibitors might be skin reactions. And we know from regorafenib that regorafenib can cause hand-foot skin reactions. Patients might get blisters wherever they have hyperkeratotic lesions on their hands or feet. So we want to make sure that these hyperkeratotic lesions that patients get rid of before they start treatment regorafenib. So we proactively send our patients to the pedicure. And with the pedicure they have to remove the hyperkeratotic lesion. And this is markedly reducing the risk for the blister formation. And we do it before patients start with regorafenib treatment. Then the patient can use special hand creams, especially with urea-based cream, so that the hyperkeratotic lesions get removed. So this is typical for the adverse events for regorafenib.
Trifluridine/tipiracil is a drug which is quite comparable to other fluoropyrimidines, so it belongs to the family of capecitabine, the IV 5-FU but also to other drugs of the fluoropyrimidines family like S-1. And these drugs might cause haemotoxicity. Haemotoxicity normally can be easily handled by oncologists. So we just have to monitor the blood cell count, but we have to make sure that we detect the low blood cell count because patients are not feeling a low level of leukocytes or low level of thrombocytes, of platelets. And so it is important that we ask the patients on a regular basis to go to the doctor or to go to the laboratory to do a blood cell count testing, and that we take actions before high grade adverse events emerge. So this is something where, especially in the time of the pandemic, we are very open to prescribe proactively G-CSF which is a colony stimulating factor, applied subcutaneously to our patients to bring up or to defuse to their white blood cell count and prevent low white blood cell levels.
And then finally, with capecitabine as I mentioned before, capecitabine also belongs to the fluoropyrimidines. And it not only can cause low blood cell count, but in addition can also cause hand foot syndrome, which is different to the hand food skin reaction, which is caused by regorafenib. So it's normally no blister formation, but in rare cases, hand and feet can get swollen. And this might be very painful. And we have to educate the patients to stop capecitabine intake when this kind of high grade adverse event emerges. And it's also important that patients on capecitabine are using hand foot creams, especially on a fairly base level. And they use these creams on a regular basis because the skin might get a very dry and then injuries can occur.
Sven De Keersmaecker
Thanks, Gerald. And so there are various treatments that we can prescribe to help manage the side effects that you've just discussed but actually one important way to improve tolerability and compliance with these medications is to employ flexible dosing of the treatments. Being flexible with the dose in response to the side effects can help the patient stay on treatment for as long as possible. Can you explain to us the approach you take to flexible dosing for these treatments and the data to support this approach?
Gerald Prager
Thank you, Sven. I think this is a really important question, especially when it comes to regorafenib. We have learnt that most potentially adverse events emerge within the first one or two cycles of treatment and especially in the time of the pandemic it is important that the patient can manage adverse events at home and they do not have to come in when adverse events emerge. So we need to prevent adverse events. So one strategy for regorafenib, for instance, is flexible dosing. What does it mean flexible dosing?
Within prospective clinical trials we have learnt that in the first cycle of treatment with regorafenib you start with an escalating regimen. So in the first week of the three weeks treatment schedule, the patients are starting with 80 milligrams per day, which is two pills per day. In the second week they go up to three pills per day and in the third week they go for full treatment in case there are no high-grade adverse events, which are four pills per day. So it is important with this escalating regimen that we have learnt that there is significantly lower probability of high grade adverse events. So the tolerability is better and, and this is very important, it's more efficient.
In the ReDOS trial - this was the name of this prospective clinical trial - we have learnt that more patients are going to cycle number three when they do the escalating regimen when compared to the conventional dosing. So it is safe and efficient to start the first cycle of treatment with a lower dose. With other clinical trials, this was confirmed with a different dosing schedule. The REARRANGE study has also shown that it is safe and efficient to start with a lower dose in the first cycle and you can eventually escalate to the full dosing in cycle two and three. Of course, if patients are suffering from adverse events, high grade adverse events, you do not further escalate the dosage so you can find the individual dose for your patients if you start with a lower dose but rapidly escalate to the individual dose the patient is tolerating. So with this escalating strategy, we have learnt to use regorafenib in a safe and efficient way.
So when it comes to trifluridine/tipiracil, trifluridine/tipiracil is as I mentioned before, a cytotoxic drug. But we normally start with the full dosage of 35 milligrams per square metre, a body surface area twice a day. And we do it the different way we would de-escalate in case of high grade adverse events or dose delays if the blood cell count on day one of the next cycle is not above that threshold. So we have more de-escalating strategy in this case. And as I was mentioning before, we are more open to use G-CSF as trifluridine/tipiracil as a cytotoxic agent.
Capecitabine as monotherapy is normally recommended in the use of 2500 milligrams per square metre made up for two dosage per day, day 1 to 14 in a three-week scale. However, normally most of the experts in most Western centres are using this drug with 2000 milligrams per square metre. So we have a bit lower dosage because we have learnt it's more safe to do so and it seems to have a comparable efficiency. However, if there are adverse events, high grade adverse events, we eventually have to step down in the dosage and go to 70% of the individual dose and second dose level for de-escalation would be 50% of the standard dosage.
It is also important that there is the recommendation that you go for a DPYD testing before you start capecitabine, because we know that patients who have a germline DPYD deficiency might suffer from high grade toxicity, which can eventually become dangerous when it goes to aplasia and neutropenic fever. So, you see, the toxicity profile is quite different. But if you take care of this, you might handle this toxicity or you can proactively prevent high grade toxicities.
So Sven, now I have a question for you. We have already mentioned the advantages of these oral treatments, but a potential disadvantage is that adherence may be reduced due to the fact that the oral drugs are self-administered. Nurses have a very important role to play here in terms of ensuring the patient understands the potential adverse events that they may experience and the importance of reporting these to their health care team so that we can advise the most appropriate strategy for managing them. Perhaps you Sven can tell us how you follow up the patients and ensure proactive reporting of adverse events by your patients.
Sven De Keersmaecker
Indeed, Gerald. As a nurse, we play an important role in the follow up of patients undergoing oral therapy. With IV chemo, you see the patients on a regular basis in hospital. With oral therapy, this is much less. As doctors and nurses, we are therefore less likely to receive information about the condition of our patients. It's important to closely monitor a patient, by controlling side effects patients can stay on their optimal treatment dose for longer. In addition, serious side effects can also affect the patient's quality of life.
After the information about the treatment has been given by the doctor, we find it important to repeat it for the patient. We check whether the patient has understood everything and whether there are any questions. We plan a telephone follow up approximately one week after the start of the treatment. We hereby inform the physician how the patient is doing, whether there are any complaints, and whether he's taking the medication correctly. In case of mild complaints, we give advice to our patients, and in case of serious complaints, we contact the attending physician and then we advise the patient in consultation with the physician.
We also do this follow up two weeks and four weeks after the start of the treatment. After that, the follow up takes place every four weeks unless the patient has to be in the hospital. Patients always receive our contact details so that they can contact us with questions or if they have complaints. We also experience that as a nurse, we are easily accessible for patients. Many patients will be less likely to report side effects to the doctor.
They are often afraid that the report will ensure that the treatment will be stopped, which is of course, not always the case. In addition, we play a key role as a nurse amongst the other members of the multidisciplinary team, we can always refer patients to the right employee; if they have nutritional problems, we send them to a dietician; if they have psychological problems, to a psychologist, etc.. Usually, this referral is only made after consultation in the multidisciplinary team.
In our hospital we also use an application online that patients can download on their own smartphone. They will then receive a notification when they should take their medication and they have the option to report parameters and any side effects. Depending on the severity of the side effects, advice is given by the application in case of mild side effects and in the event of a serious side effects, an email will be sent to the nurse and to the oncologist. They will then contact the patient for further information. We've been using this application for several years and patients are very satisfied with it. They have the feeling that they can always report the complaints and that follow up is ensured. Moreover, they think it's positive that they receive a reminder when they have to take their medication correctly as well as the correct dose.
As you highlighted, Gerald, we can instruct the patients to take a certain dose of their oral medication at home. But ultimately we have to trust that they take it as instructed. There is a risk, of course, that if patients are struggling with side effects, that they might stop the treatment temporarily, but not report this to their health care team. I'm curious whether there is any other way we can check patients’ compliance, for example, through blood tests?
Gerald Prager
Yes Sven, you're absolutely right, especially when it comes to capecitabine, we can look at the blood cell count and the red blood cell count and normally on especially long intake of capecitabine what we observe is the mean corpuscular volume, so the MCV level is increasing. So capecitabine is leading to a microcytosis. And so we can look at this and we know whether the patient is compliant or not. But you were absolutely right. I think it's very important also to hand out patient diaries where they can fill in adverse events and of course, that they have taken the oral drug. It is important also on our side the informed consenting that the patient get confident with the oral drug treatment. And in my experience, it is very important to take the time in patient education as the attachment to the treatment is much higher than if you just prescribe a drug. So I think it's extremely important the interaction of the nurses with the patients to follow up the compliance.
Sven De Keersmaecker
Okay. Thank you, Gerald. We've had a great discussion today. Perhaps you could summarise the key points for our listeners.
Gerald Prager
Sure. So I think it is important that in the year 2022, we have oral drugs available for our patients in the treatment of metastatic colorectal cancer. It's convenient for the patients. They can take the drugs at home. It is also safe for the patients because they do not have to come in that often on day clinics in the time of the pandemic. However, it is important for us to find an individual dose for the patients, and we have learnt within the last months by prospective clinical trials that it is safe to start with an escalating regimen when it comes to regorafenib and to do a blood cell monitoring when it comes to cytotoxic drug like capecitabine or trifluridine/tipiracil.
It is also extremely important that nurses have tight interactions with our patients when on treatment with oral drugs. And so I can only express how much I appreciate the good collaborations between doctors and nurses on this matter.
Sven De Keersmaecker
Well, thanks again, Gerald, and thanks to our listeners. We hope you have found our discussion useful.
Gerald Prager
Thank you very much Sven. And thank you very much to all listeners.








 Downloadable
Downloadable  5 MIN
5 MIN
 Jun 2025
Jun 2025