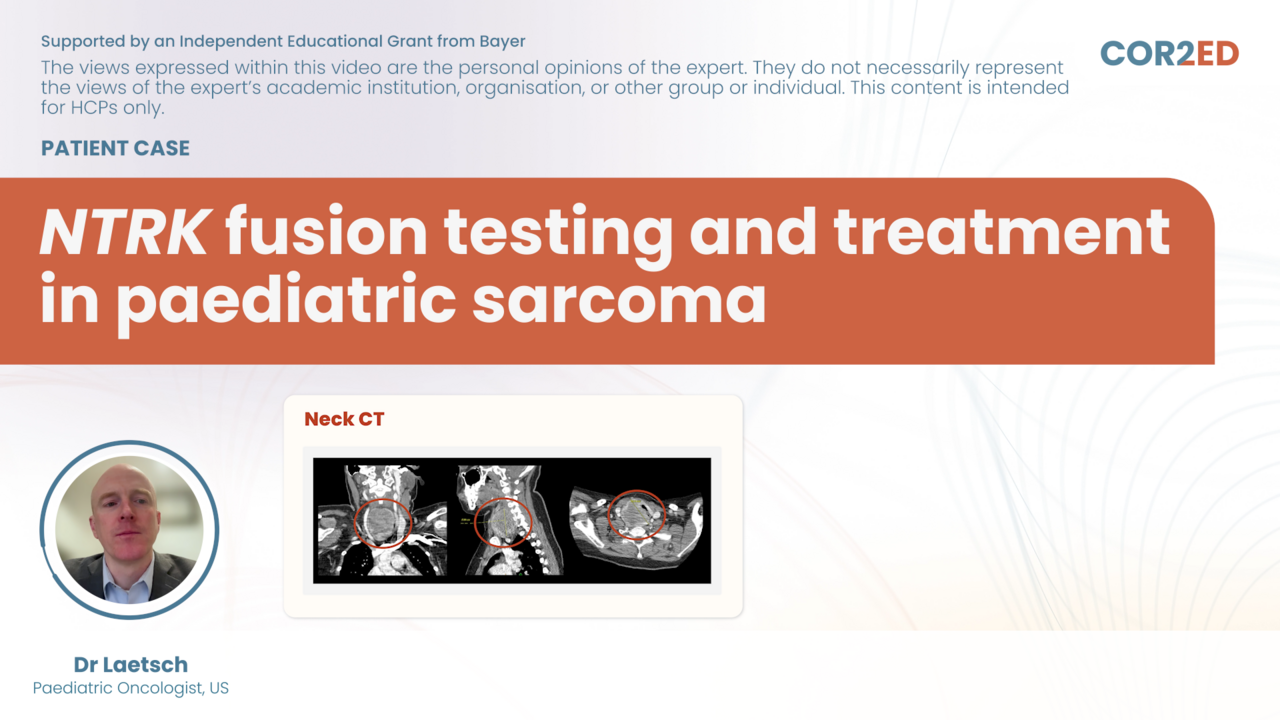
Podcast: Timing of ARSI and taxanes for mCSPC
Please note:
GU CONNECT podcasts are designed to be heard. If you are able, we encourage you to listen to the audio, which includes emotion and emphasis that is not so easily understood from the words on the page. Transcripts are edited for readability. Please check the corresponding audio before quoting in print. This GU CONNECT programme is supported through an independent educational grant from Bayer is an initiative of COR2ED. The views expressed within this podcast are the personal opinions of the authors. They do not necessarily represent the views of the author’s academic institution, or the rest of the GU CONNECT group.
Transcript
Tanya Dorff
Hello, and welcome to this podcast on timing of androgen receptor signaling inhibitors and taxanes for metastatic castration-sensitive prostate cancer. I'm Dr Tanya Dorff, a medical oncologist from the City of Hope Comprehensive Cancer Center and I'm joined today by Dr. Neal Shore. Neil, perhaps you can introduce yourself.
Neal Shore
Yeah, so thanks so much Tanya, what a great pleasure to talk with you today on this really important topic, the timing of the ARSI and taxanes in mCSPC. A lot has been forthcoming in our congresses and publications. You know, I'm a Uro-Oncologist, I practice in South Carolina, I'm part of Genesis Care US and I run our research center Carolina Neurological Research Center.
Tanya Dorff
Well, I'm really excited to have the conversation with you also, I think you have such a great way of representing what's happening among urologists and urologic oncologists who treat these patients, as well as me bringing the medical oncology perspective.
So today we're talking about metastatic castration-sensitive prostate cancer. Especially looking at some of the newer data presented at GU ASCO and ASCO this year, to address when to use intensified therapy with androgen receptor signaling inhibitors and taxanes.
The treatment landscape has really changed significantly over the last seven years. It started with intensification with docetaxel from CHAARTED and STAMPEDE and then more recently abiraterone in LATITUDE and STAMPEDE and then apalutamide in TITAN, enzalutamide in ARCHES and ENZAMET.
And now, this past year we've seen data on triplet therapy from PEACE-1 and ARASENS.
Neal Shore
It's so great, I think, for a medical oncologist and a urologist, representing our experiences in academia and the community, to talk about how to optimise mCSPC therapy.
For decades, and decades and decades, right we've been all about monotherapy since Huggins and Hodges days, and we pay homage to them all the time. But let's put it right up front for our colleagues listening - monotherapy, ADT alone, ADT alone, whether it's an agonist and antagonist, is almost never the right decision anymore, because of these amazing trials that you've outlined Tanya.
I mean this is level one evidence that's now been incorporated into the guidelines that couplet therapy, and we're going to talk about triplet therapy today, how important it is in optimising patient care by not only delaying the conversion to resistance when we know the biology starts to really hasten because of clonal mutations of populations, but it, not only with resistance, we see you know, a rapidity towards a demise and worsening radiographic progression and all the usual secondary endpoints.
So tremendous work that was done in the last year, in less than a year, we saw the presentation of PEACE-1 by Karim Fizazi at ESMO 2021 and then at ASCO GU 2022 the presentation by Matthew Smith on ARASENS.
And what's really remarkable is, if you say, well, if two is better than one, couplet better than monotherapy, could triplet therapy be better than couplet therapy? And that's an ongoing controversy and it'll be fun to have this conversation today, looking at the data.
And so, I think it's really rather amazing that we still have a lot of our colleagues who are doing monotherapy. And part of the reasoning, maybe, maybe they can't get access to drugs, the approved therapies, the ARPIs, taxanes. Maybe it's they see the PSA go down and say well that's good enough. Maybe they're uncomfortable with the toxicity management. Maybe it's an economics issue. I mean all of these things are not unreasonable, but I think at the end of the day we must optimise patient care and I think this is true, all over the world. Do you agree with that?
Tanya Dorff
Yeah, I think that's a great point to emphasise that, it's been years that we've known doublets are better than monotherapy and yet uptake has been slow. It's certainly improving over time but it feels like there's going to be a big hurdle to get people to think about triplets. And especially because both PEACE-1 and ARASENS really assumed everyone gets docetaxel and the question that was being asked was whether or not you still need the intensified androgen receptor agent, you know, the case of PEACE-1 abiraterone and in the case of ARASENS darolutamide.
But it still doesn't answer the question of whether you need the docetaxel if you are already planning to use the intensified androgen receptor agent. So, both studies are limited by that design and I think has led to some concern about how much uptake there will be, given that we're just getting people used to doublet and we're not so sure what the addition of the taxane really adds.
I mean, aside from the fact that, of course, both studies were absolutely positive. I mean the primary endpoint of PFS in PEACE-1 was achieved in addition to a 25% improvement in overall survival when you add abiraterone after someone gets those six doses of upfront docetaxel.
And, similarly, in ARASENS, you know, a 32.5% improvement in overall survival for using darolutamide on top of those six doses of docetaxel. But we have some work to do in educating folks in terms of the benefits. You know Steve Freedland presented a poster that I thought was fascinating at ASCO asking that question, 'why don't you use doublet therapy'? And some people still feel like, 'I'll keep it in my back pocket', but I think all these trials clearly show that's not the right approach.
So, what do you think stands out from PEACE-1 and ARASENS?
Neal Shore
Well, I am a believer in the concept of applying as many novel mechanisms of action that can thwart the cellular proliferation of adenocarcinoma cells in a timely way. I would also suggest that your first shot on goal, to use a sports analogy, tends to be your best shot on goal. Patients typically present with de novo mCSPC which really was everybody in the PEACE-1. ARASENS was about 85% de novo and about 15% recurrent.
We know that recurrent patients or what some people call metachronous as opposed to synchronous, I like de novo and recurrent, it seems a little clearer to me, but that the recurrent or the metachronous, maybe it's not as an aggressive biology.
That's sort of a difference and how the studies were applied, but I like the idea of combining novel mechanisms of action.
These patients tend to be younger than our CRPC patients, they tend to be in a better performance status. It's only six cycles which is over in four months, but I understand where some of our colleagues will say 'yeah it's still chemotherapy, there are risks of toxicity'. So, for example in PEACE-1 we saw maybe 6% of transaminitis or LFT elevations compared to 1% in the doublet versus the triplet arm. A little bit more hypertension 22% versus 13%.
ARASENS - the reported AEs we're pretty comparable in both arms, pretty balanced. The serious adverse events were 44.8 versus 42.3 in the triplet versus the doublet. Any one of the things about daro(lutamide) and abiraterone, these drugs have been really pretty well characterised the last several years.
I personally find docetaxel in this population to be well-tolerated, but I think you're right Tanya, there are some of our colleagues who say, ‘it's still chemotherapy, it's a taxane, there's maybe this risk of myelosuppression, febrile neutropenias’.
I guess I go back to, you know when you have high-volume disease, I guess I'd like to ask you, do you in your mind have a difference in the way you look at a patient when you're in the clinic if it's 4 bone lesions versus 15 or 20 bone lesions or, certainly if it's a patient who has liver metastases or even maybe a patient who has really high-volume lymphadenopathy, and that's technically low volume disease but is really mediastinal, big retroperitoneal pelvic nodes. Do you kind of delineate your high-volume stratification?
Tanya Dorff
That's a great point you raise. I think we have work to do to really define high-volume or high-risk, you know, there are different definitions that have been applied in different studies.
And I think some of it does come down to clinical intuition. I do think very bulky lymph nodes are worrisome, that means there's a lot of cancer that could have mutated or de-differentiated and that's where I start to think about different mechanism of action like you pointed out.
Maybe not all of the cancer is as hormonally driven. And it feels like we're going to achieve a better remission by applying the taxane which kills the faster growing maybe more aggressive clones. And I think it's appealing, but you know for medical oncologists it kind of makes sense, we're used to sort of induction and consolidation or maintenance and that's kind of how this can be viewed. But I think, maybe from the urology perspective, there is a little more concern about chemo toxicity.
I also feel like there are those patients that just, your gut tells you that this is a patient who needs it. Whether it's something in the pathology report showing, maybe some neuroendocrine differentiation or the genomics coming up with aggressive variant signature or a low PSA with a high-volume of disease, that always definitely makes me worry, since we know PSA is to some extent a representation of androgen receptor signaling.
So, I feel like there are definitely these patients in clinic that jump out as needs a taxane. I wonder, for you are there specific things where you would say absolutely no taxane, like is it an age issue or a comorbidity issue?
Neal Shore
I agree. The fun part about what we still continue to do is the art of medicine and yeah, I try not to be overly biased by chronologic, biologic age. I look at the performance status, and as we see the graying of the world, sometimes, you know, 80 is the new 60.
And, of course, I don't say that completely tongue in cheek, but you have an 80-year-old, who is fit, who's chemo fit, who is suddenly showing up at your door with de novo metastatic disease and has good actuarial survival.
I would consider giving that patient ADT doce(taxel), and then adding abi(raterone) and/or daro(lutamide) based upon this data.
That said that's probably more the exception than the rule and you're right if people are frail and if people are younger and have lots of co-morbidities, borderline renal insufficiency, diminished cardiac performance. You know I'm going to probably be more comfortable in recommending just couplet.
But you know, I think that this data, because it's such strong data and such well-done studies, PEACE-1 and ARASENS, it really is incumbent upon us to have that vaunted shared decision-making conversation.
Tanya Dorff
Yeah, I was just thinking about how well represented older patients are, because there is a difficulty that's been recognised inadequate representation of geriatric patients in clinical trials.
The ARASENS, though, did have at least 15% of patients who were over 75, though.
Whereas PEACE-1, I think the oldest patients were in their 70s, early 70s, so... but at least from ARASENS we can say these are a good percentage of patients who are in that older age group, and because they had adequate performance status, like you pointed out, they were able to tolerate the docetaxel without excessive toxicity seen in that study.
You know and there's some previous publications on taxanes showing that it is tolerable in geriatric populations as well, so I hope others will also think like you and look more at who the patient in front of them is, and not just sort of the numbers.
What about access to these therapies? Has that been an issue in your region?
Neal Shore
Well, that's a great question. I think, and we think about our colleagues globally. I mean docetaxel is generic, and it's been generic for quite some time so there's less of an economic accessibility issue.
Abiraterone has gone generic for much of the world. Darolutamide is not, and I think, for the most part, other ARPIs are not as well, so the economic accessibility becomes a challenge.
For me, even prior to PEACE-1 and ARASENS…let me ask you this question. So, if you had a patient who came in to see you, four years ago, who was 60 years old in great health, triathlete, family history of cancer. You did genomic profiling, didn't have a homologous recombinant repair alteration, wasn't MSI high but had a few liver metastases and a few bone lesions and some lymph nodes. Totally asymptomatic just picked it up on a on a screening PSA and if, in fact, you said 'I'm going to hit you with the ADT and docetaxel because of the liver lesions'. At that point would you've just stopped and not all offered that patient and ARPI and if the patient, especially said to you, 'hey I want to just give me everything your got, I want the kitchen sink'?
Tanya Dorff
Well, I think, a few years ago we didn't have the data. Of course, it was tempting because it feels a little bit incomplete, right? After you give the six doses of docetaxel and now, you're just maintaining on castration although that is how we practised for a long time, is as you mentioned monotherapy.
And I guess there's always that question about how well the androgen receptors signaling inhibitors function in someone like you described with visceral metastases. Although I would say some of the early studies did show efficacy, even in visceral populations, but I hope that everyone's evolved to really just feel that that single castration therapy feels inadequate in the setting of metastatic disease.
And that the earlier we intensify, the earlier we use our drugs, the more we get out of them. You know it's been so striking to me that despite crossover we see this profound survival advantage which really tells us these drugs need to be used earlier to have the greatest impact.
And I think back to how my patients used to really progress much more rapidly and symptomatically and people worry, are we driving this crazy neuroendocrine differentiation? Are we driving more aggressive variants? But that's not what we're seeing. What we're seeing is just people living a lot longer.
Like the SWOG study S1216 where even the control arm is just doing so much better. I think all of our treatments have really improved outcomes but using them early is so profoundly impactful.
So, what do you think, in terms of progression now? How is this all this shifting in the upfront setting going to change how we approach castration-resistant disease?
Neal Shore
Yeah, and I loved your last point and data now when you look at 2009 where the average asymptomatic mCRPC patient lived 19 months, now we're seeing asymptomatic mCRPC patients living three and a half to seven years.
We've made great strides but it's still a lethal disease so again, I kind of go back to give everything you can do in a rational way with novel mechanisms of action. So yeah, when you get to resistance disease and the biology is more aggressive at that point just by definition. More clonal mutations. Of course, I love the notion that everybody should be getting routinely, as long as they economically and methodologically we can get access to both germline and somatic alterations, if there is a DDR DNA damage repair alteration, certainly BRCA throughout the world, arguably, even ATM and a whole list of other gene alterations in that pathway plus MSI high.
These are fantastic precision-based opportunities for therapy. If it's bone dominant disease there's the opportunity for a drug, such as radium-223. And of course, we have, if they didn't receive a taxane we've got two really effective taxanes in docetaxel and cabazitaxel.
Now we have the PSMA-RLTs, you know, with the first approval coming from lutetium 617.
More and more to come, parts of the world, Germany, Australia, they have both beta and alpha particle-based PSMA-RLTs. And it's only a matter of time where that will become more accessible.
So, this concept is: A, do genomic profiling; B, look at the tumour burden. Your point about neuroendocrine and small cell and poorly differentiated, big unmet need, patients with RB1 loss. Those kinds of patients maybe do much better with combination carbo-platinum and a taxane, and of course clinical trials.
But without making it too complicated, one point I'd love to make is that we've done studies and we see that the average patient who succumbs in 2019 received on average 2 life-prolonging agents and we just got through listing 12 life-prolonging agents, with 7 novel mechanisms of action. So, I think that's implied in your question that, we have so many opportunities to offer our patients better care, we have to be really judicious and optimising the sequencing.
Tanya Dorff
I love that you raised clinical trials, because, as you mentioned there's still work to be done, and sometimes the best treatments that are out there are on clinical trials right now. We have these really exciting agents coming down the pipeline and often they're restricting the number of prior lines of therapies so thinking about clinical trials early is always a good strategy, just like intensifying early is.
So, any final comments as we wrap up our discussion today?
Neal Shore
Yeah, I think that your comments and your insights, I'm in 100% agreement. I really believe that the complexity of advanced prostate cancer care is a wonderful thing because it requires a multidisciplinary team. The multidisciplinary team now is essential to making sure our patients get the best care. Medical oncology, uro-oncology, radiation oncology, nuclear radiologists, pathologists, genetic advice and counselling or the very least, if you don't have a GC get educated and offer our patients as many opportunities to fight this disease.
And we've made great progress, I guess it still is going back to the beginning of our presentation: monotherapy no, couplet therapy absolutely, triplet therapy for a certain segment of the population in my mind, undoubtedly.
And then we have many other approved agents once they develop resistance.
Tanya Dorff
Thanks so much for that great summary and thanks again for joining me today for this podcast.
Neal Shore
Thanks Tanya.








 Downloadable
Downloadable  5 MIN
5 MIN
 Jun 2025
Jun 2025