NTRK CONNECT has developed a comprehensive resource, available at ESMO OncologyPRO, providing information on TRK fusion proteins as a therapeutic target for specific patients across multiple tumour types. The content was first developed in 2019, it has been updated in 2022 to provide the latest information and a greater focus on four tumours: lung cancer, gastrointestinal cancer, thyroid cancer and sarcoma. The content is published on OncologyPRO: The home of ESMO’s educational and scientific resources.
TRK Fusion-Positive Cancer
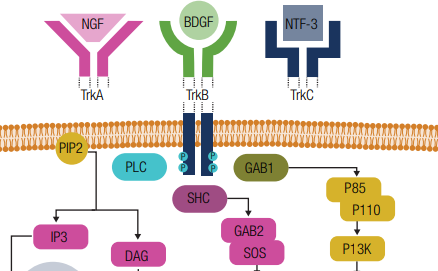
This learning resource has been developed to provide information on the detection and treatment of TRK fusion-positive cancer. The material has been compiled from a review of the medical literature and expert guidance from a scientific committee comprising international experts in the fields of pathology and medical oncology.
There are three modules covering:
- An overview of cancers with NTRK gene fusions
- The importance of testing for cancers with NTRK gene fusions
- A detailed summary of NTRK gene fusions in selected cancers, namely Lung Cancer, Gastrointestinal Cancer, Thyroid Cancer and Sarcomas