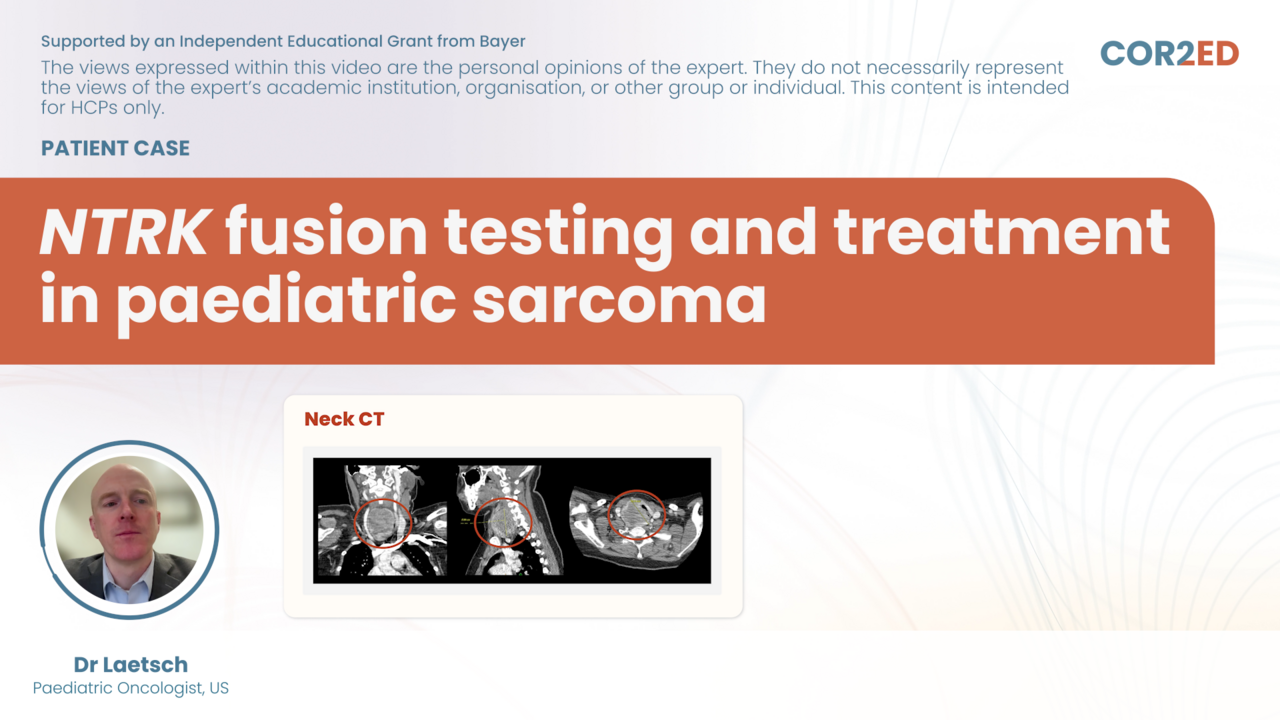
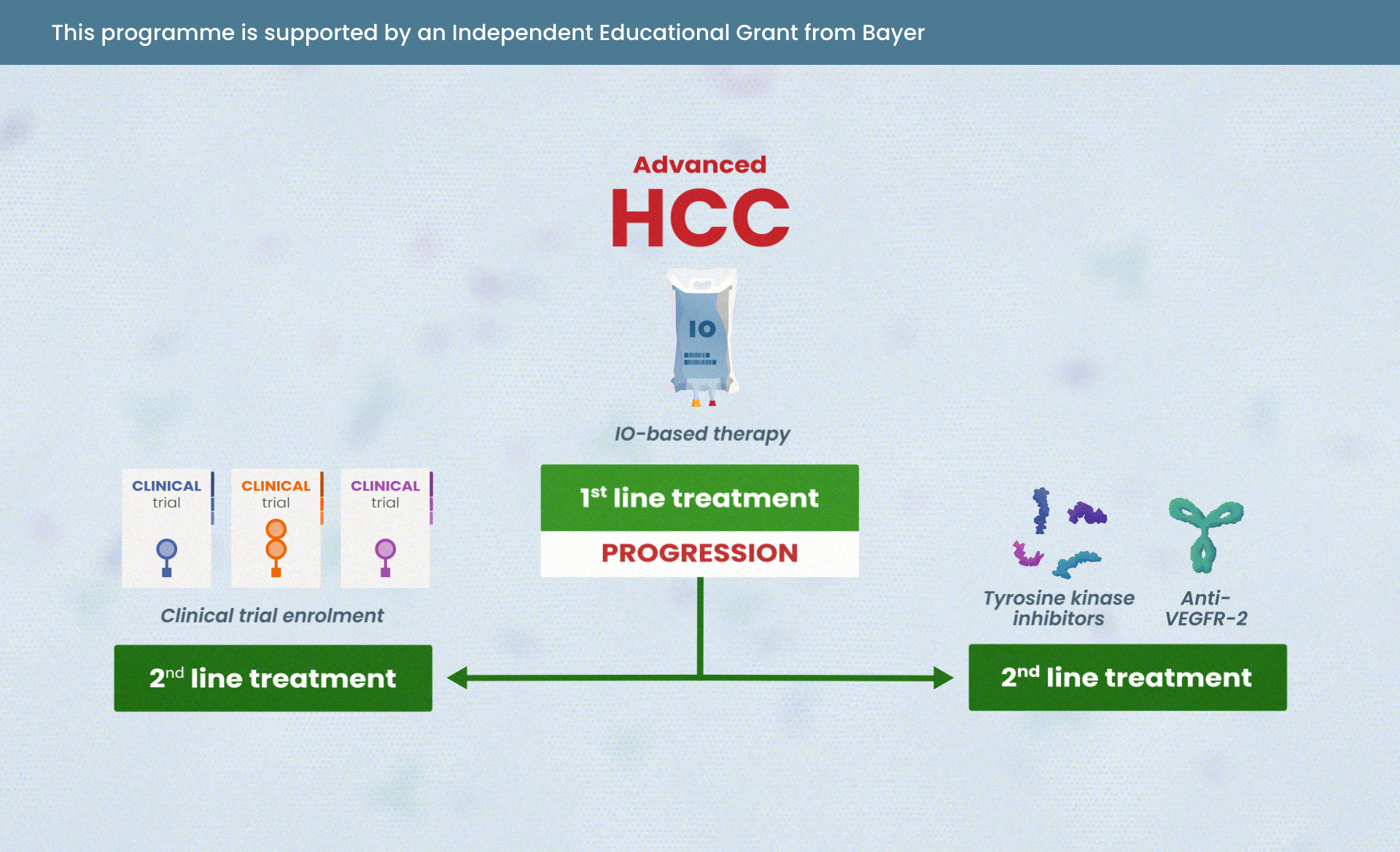
BMedSci, BMBS(Nottm), MRCP(UK), FAMS Dr Choo Su Pin is a medical oncologist at Curie Oncology who specializes in gastrointestinal cancers. She graduated from the University of Nottingham Medical School, UK before returning to Singapore. She has fellowships from the Royal College of Physicians, UK and the Academy Of Medicine, Singapore and was trained in medical oncology at the National Cancer Centre Singapore (NCCS) where she was Chief of Gastrointestinal Oncology. She also did a research fellowship at the University of California San Francisco. Her research areas of interest include pancreatico-hepatobiliary cancers and early phase trials. Relevant publications: Wong DJ, Lee J, Choo SP et al. Hyperprogressive disease in hepatocellular carcinoma with immune checkpoint inhibitor use: a case series. Immunotherapy 2019 Feb;11(3):167-175 Koh YX, Tan HL, Lye WK, Kam JH, Chiow AKH, Tan SS, Choo SP, Chung AYF, Goh BKP. Systematic review of the outcomes of surgical resection for intermediate and advanced Barcelona Clinic Liver Cancer stage hepatocellular carcinoma: A critical appraisal of the evidence. World J Hepatol 2018 Jun 27;10(6):433-447 Lim HY, Merle P, Weiss KH, Yau T, Ross P, Mazzaferro V, Blanc JF, Ma YT, Yen CJ, Kocsis J, Choo SP et al. Phase II studies with refametinib or refametinib plus sorafenib in patient with RAS-mutated Hepatocellular Carcinoma. Clin Cancer Res 2018 Oct 1;24(19):4650-4661 Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, Choo SP et al; Asia-Pacific Hepatocellular Carcinoma Trials Group. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol 2018 Jul 1;36(19):1913-1921. Teo JY, Allen JC, Ng DCE, Abdul Latiff JB, Choo SP et al. Prospective study to determine early hypertrophy of the contra-lateral liver lobe after unilobar, Yttrium-90, selective internal radiation therapy in patients with hepatocellular carcinoma. Surgery 2018 May;163(5):1008-1013 Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, El-Khoueiry A, Kelley RK, Borbath I, Choo SP et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol 2018 Jan 20;36(3):276-282 Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, Choo SP et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov 2017 Oct;7(10):1116-1135 El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017 Jun 24;389(10088):2492-2502 Zhai W, Lim TK, Zhang T, Phang ST, Tiang Z, Guan P, Ng MH, Lim JQ, Yao F, Li Z, Ng PY, Yan J, Goh BK, Chung AY, Choo SP et al. The spatial organization of intra-tumour heterogeneity and evolutionary trajectories of metastases in hepatocellular carcinoma. Nat Commun 2017 Feb 27;8:4565 Chay WY, Tham CK, Toh HC, Lim HY, Tan CK, Lim C, Wang WW, Choo SP. Coriolus versicolor (Yunzhi) Use as Therapy in Advanced Hepatocellular Carcinoma Patients with Poor Liver Function or Who Are Unfit for Standard Therapy. J Altern Complement Med 2017 Aug;23(8):648-652. Tai WM, Yong WP, Lim C, Low LS, Tham CK, Koh TS, Ng QS, Wang WW, Wang LZ, Hartono S, Thng CH, Huynh H, Lim KT, Toh HC, Goh BC, Choo SP. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann Oncol 2016 Dec;27(12):2210-2215 Choo SP, Tan WL, Goh BK, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016 Nov 15;122(22):3430-3446. Chan-On W, Nairismägi ML, Ong CK, Lim WK, Dima S, Pairojkul C, Lim KH, McPherson JR, Cutcutache I, Heng HL, Ooi L, Chung A, Chow P, Cheow PC, Lee SY, Choo SP et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet 2013 Dec;45(12):1474-8









 Downloadable
Downloadable  5 MIN
5 MIN
 Jun 2025
Jun 2025