In Module 1 of this micro learning, developed by experts Prof. Peter R. Galle and Dr Aiwu Ruth He, you'll learn about the safety and efficacy of immunotherapy (IO) for hepatocellular carcinoma (HCC) and how to implement IO treatments for patients with HCC in clinical practice.
The content includes a short animated video, downloadable resources, and an assessment to test your knowledge.
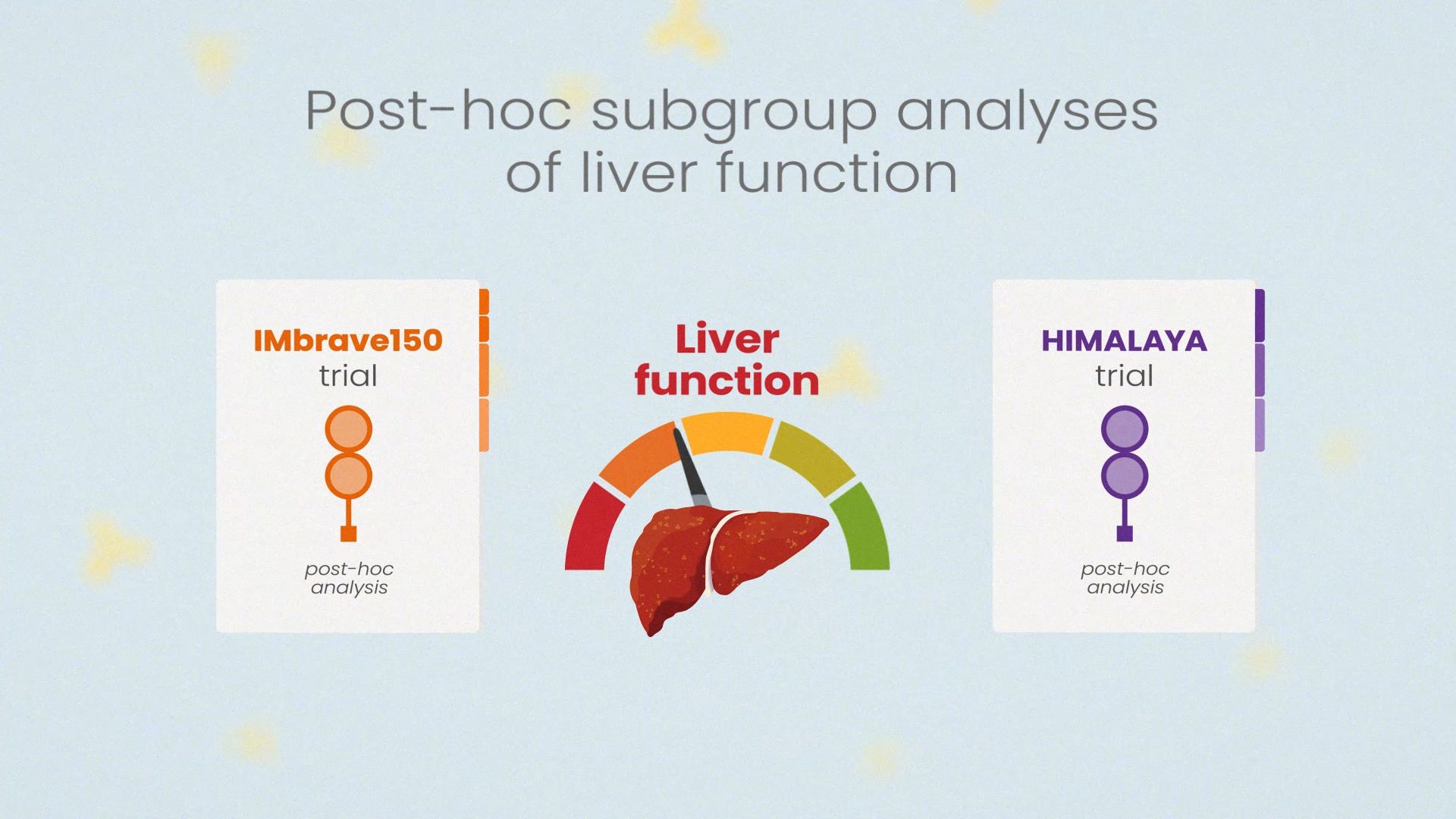
When you're ready, move on to Module 2: The Use of Immunotherapy in HCC: In-depth Subgroup Analyses and Challenges.
Efficacy of IO for HCC
Learn how to:
- Recognise the efficacy of IO and IO combinations
- Understand the survival curve when assessing IO treatment
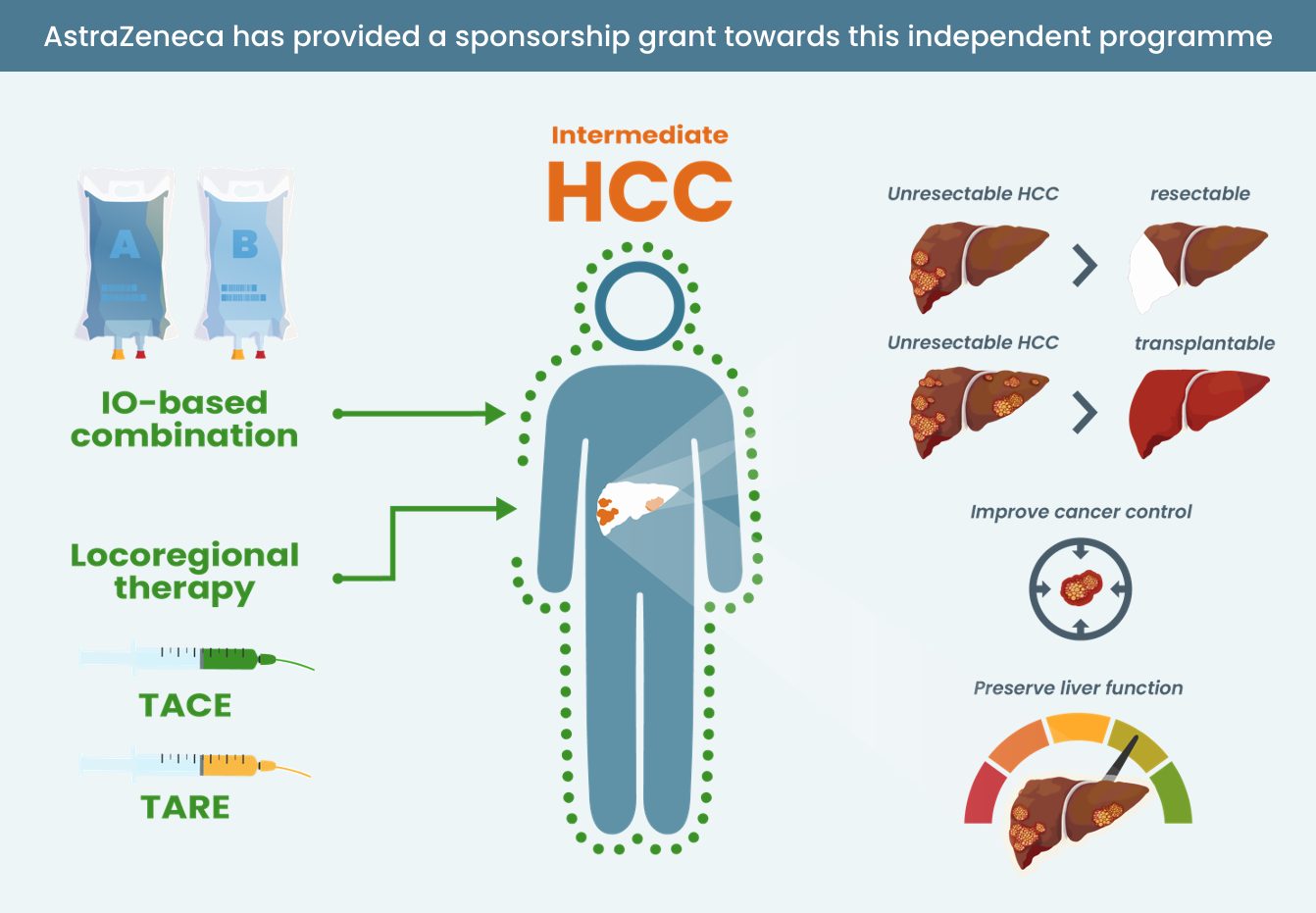
- Know where IO and IO combinations sit in the treatment landscape for patients with HCC
Safety of IO for HCC
Learn how to:
- Recognise the immune-related adverse events (irAEs) and potential additive or synergistic toxicities associated with IO combinations
- Know how to identify and manage these AEs early on
Clinical Takeaways
-
IO, and in particular IO combinations, are effective treatments for patients with advanced HCC
-
Survival analysis for IO treatment shifts from median overall survival (OS) to landmark analysis
-
IO and IO combinations may result in inflammatory side effects, known as irAEs. Most of these AEs can be treated with steroids











 Downloadable
Downloadable  5 MIN
5 MIN
 Jun 2025
Jun 2025 





