Following on from Module 1, The Use of Immunotherapy in HCC: Efficacy and Safety, you can now go deeper with your learning in Module 2, The Use of Immunotherapy in HCC: In-depth Subgroup Analyses and Challenges, brought to you by HCC experts Prof. Peter R. Galle and Dr Aiwu Ruth He.
In this module, you'll learn about current data and the efficacy and challenges of immunotherapy (IO) for different subgroups of patients with hepatocellular carcinoma (HCC), including how to:
- Assess liver function using the Albumin-Bilirubin (ALBI) scoring system to evaluate a patient's eligibility for IO treatment
- Understand the efficacy and safety data in the different subgroups eligible for IO treatment
Watch the 4-minute video and download the accompanying slides and flashcard for your reference.
When you're ready to test your knowledge, take the assessment using the blue button beneath the video.
Clinical Takeaways
-
The Child-Pugh and ALBI scoring systems are methods to assess liver function
-
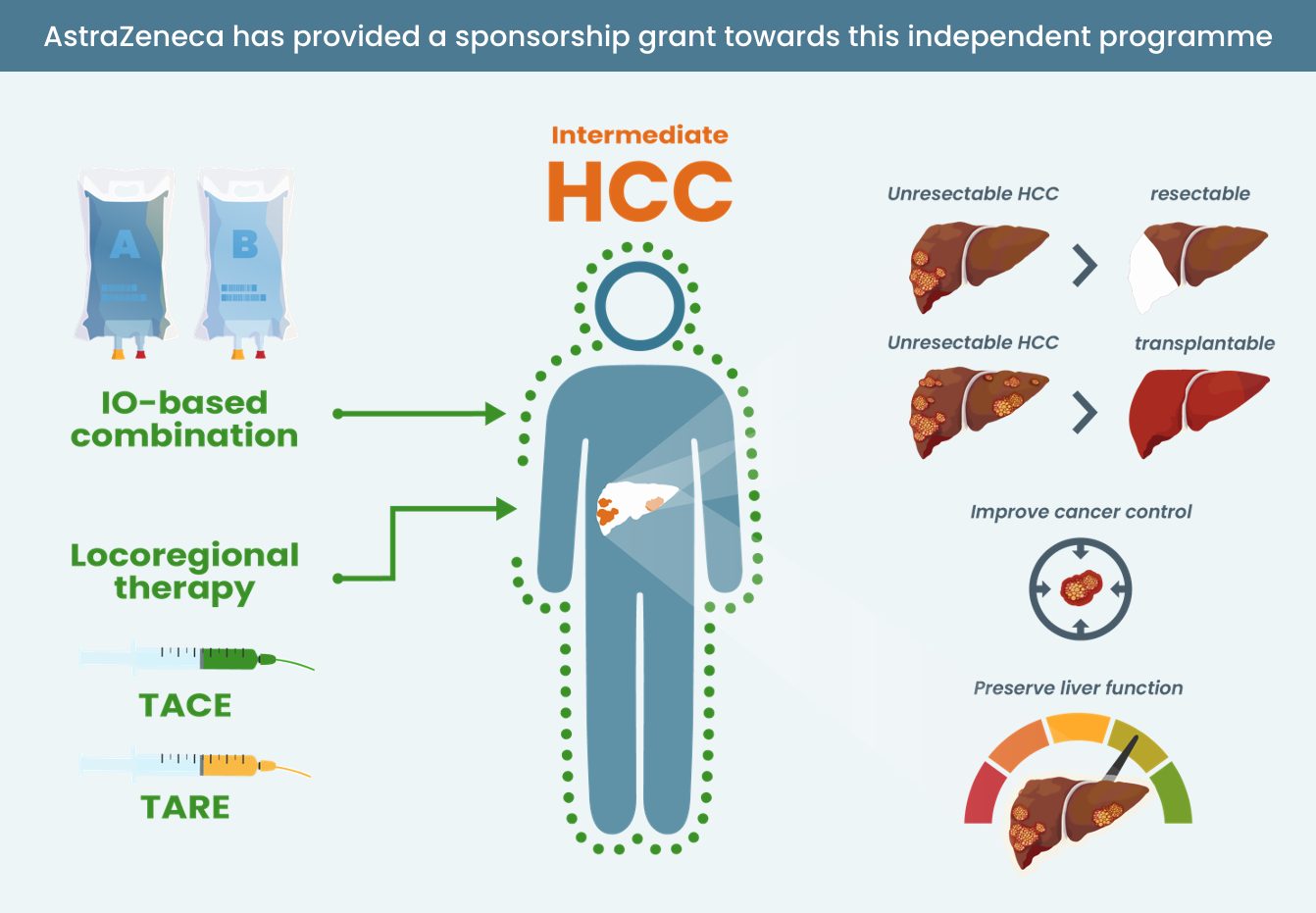
There is a need to understand whether specific groups of patients with HCC benefit more from one systemic treatment than another
-
Subgroup analyses in patients with HCC receiving IO and IO combinations show variable hazard ratios across etiologies and liver function. These data are not mature enough to guide treatment decisions










 Downloadable
Downloadable  3 MIN
3 MIN
 Mar 2026
Mar 2026 





