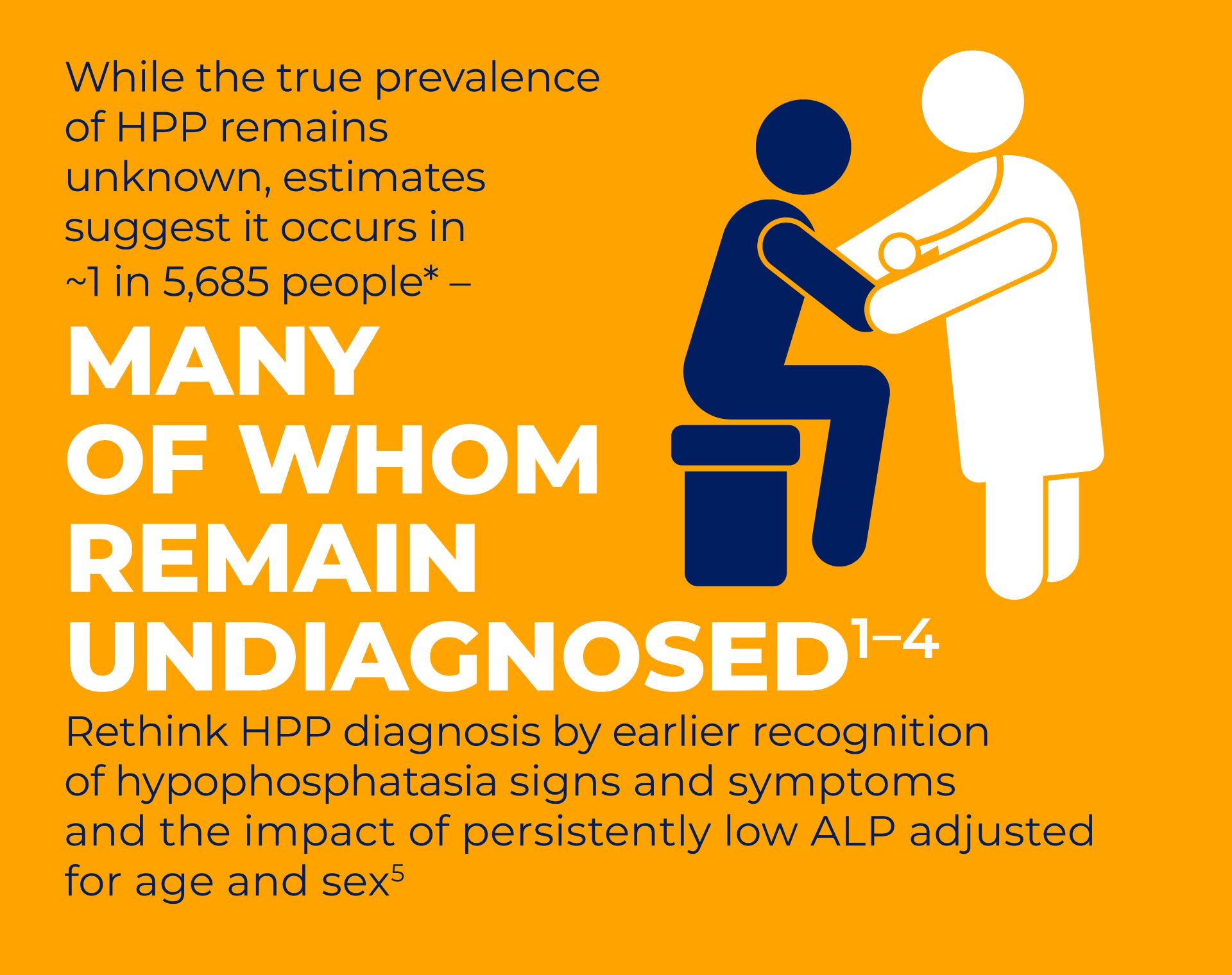
View each of the 5 fact cards to reveal key statistics and clinical insights on the diagnosis and disease burden of HPP in children and adults.
Will they make you rethink HPP diagnosis and disease burden?
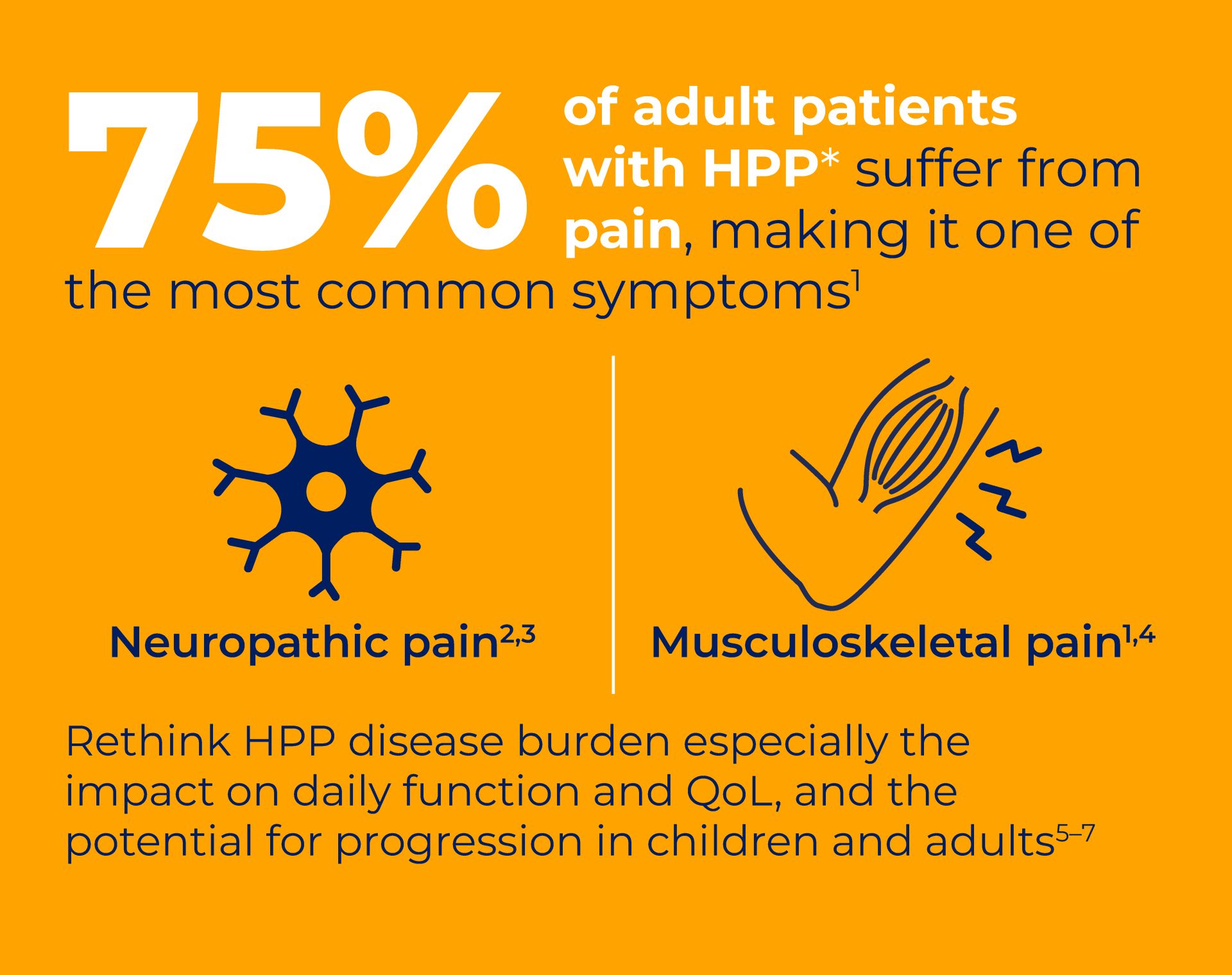
View each of the 5 fact cards to reveal key statistics and clinical insights on the diagnosis and disease burden of HPP in children and adults.
Will they make you rethink HPP diagnosis and disease burden?
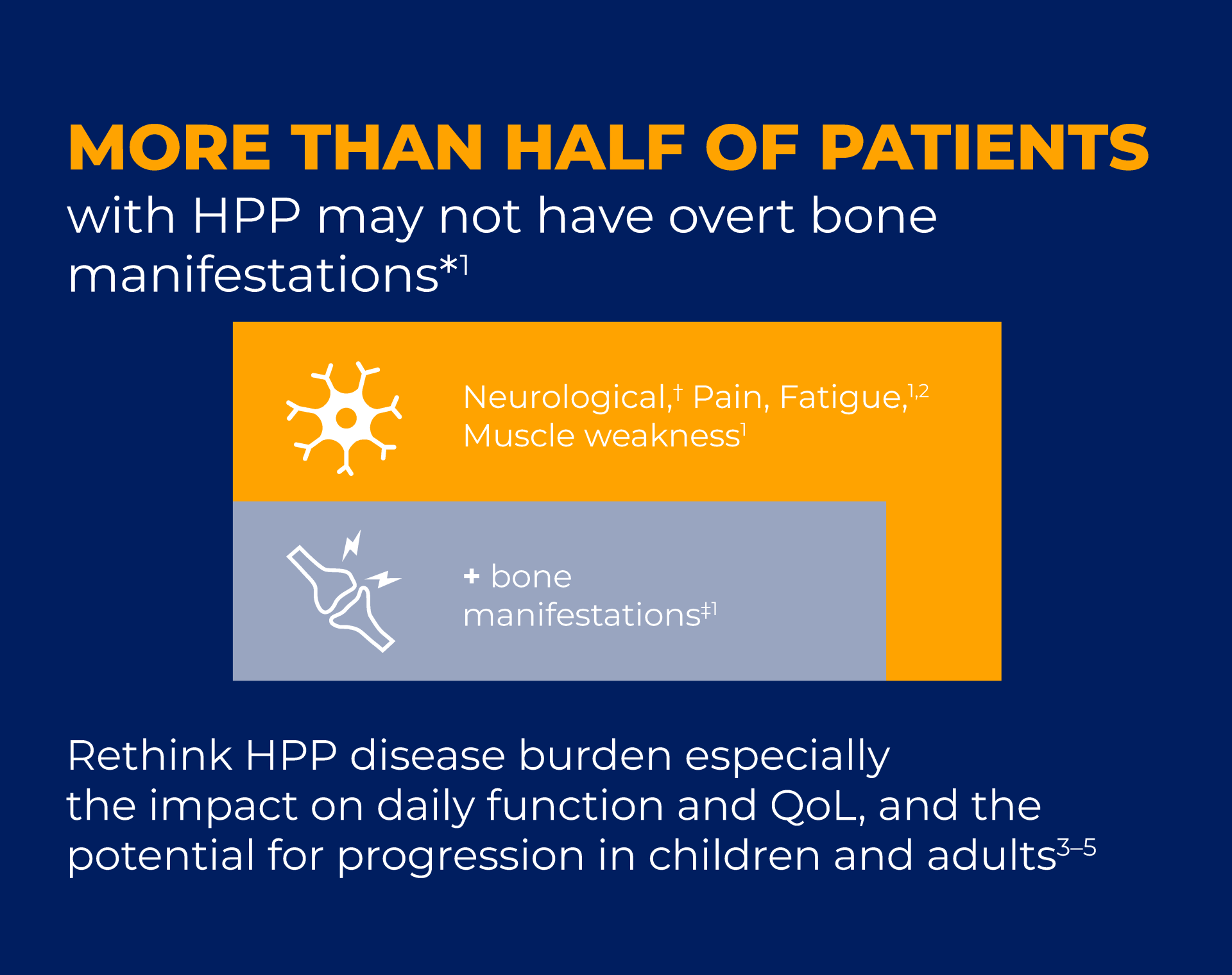
View each of the 5 fact cards to reveal key statistics and clinical insights on the diagnosis and disease burden of HPP in children and adults.
Will they make you rethink HPP diagnosis and disease burden?
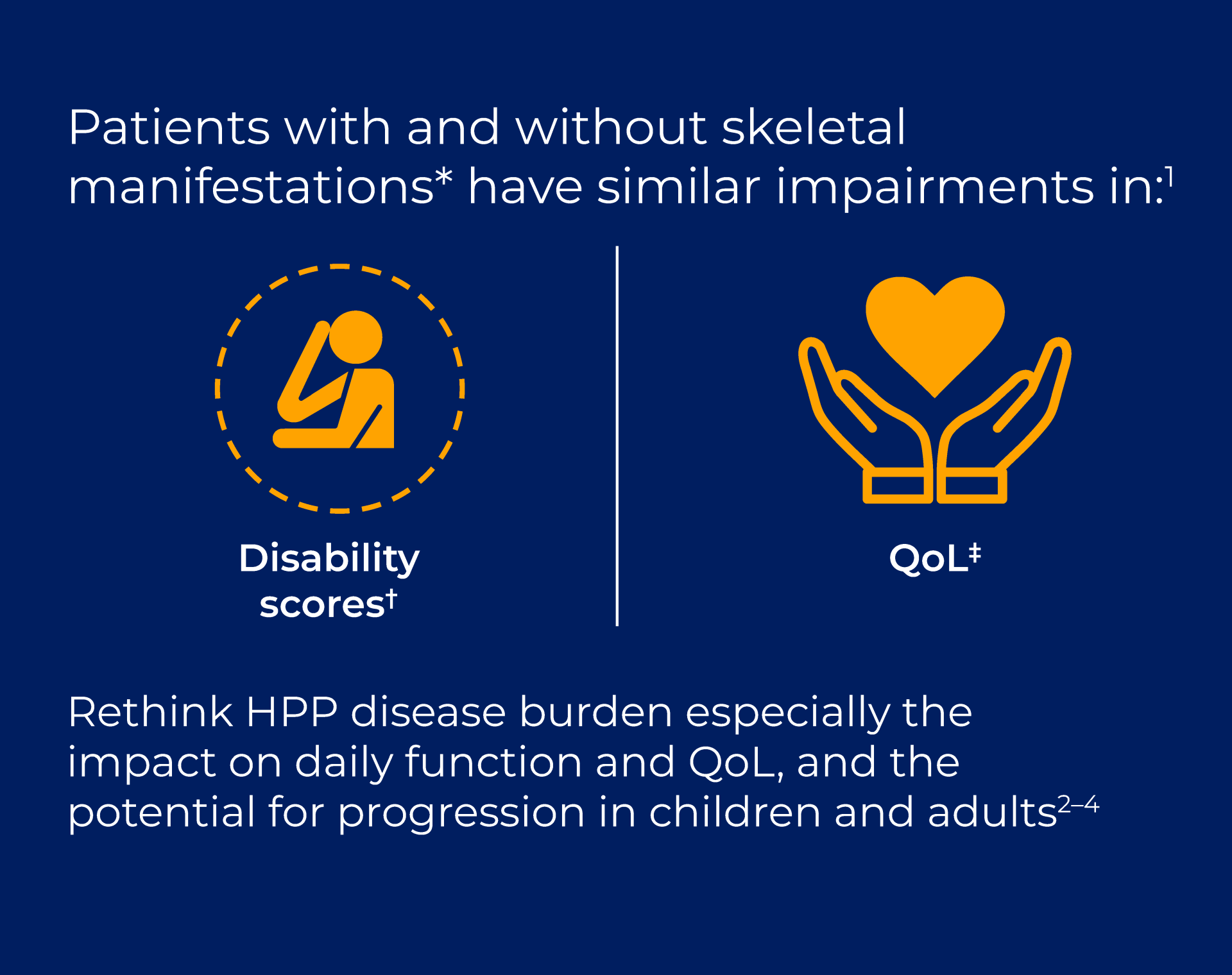
View each of the 5 fact cards to reveal key statistics and clinical insights on the diagnosis and disease burden of HPP in children and adults.
Will they make you rethink HPP diagnosis and disease burden?
View each of the 5 fact cards to reveal key statistics and clinical insights on the diagnosis and disease burden of HPP in children and adults.
Will they make you rethink HPP diagnosis and disease burden?



 2 MIN
2 MIN
 Jun 2025
Jun 2025 

 Downloadable
Downloadable 




