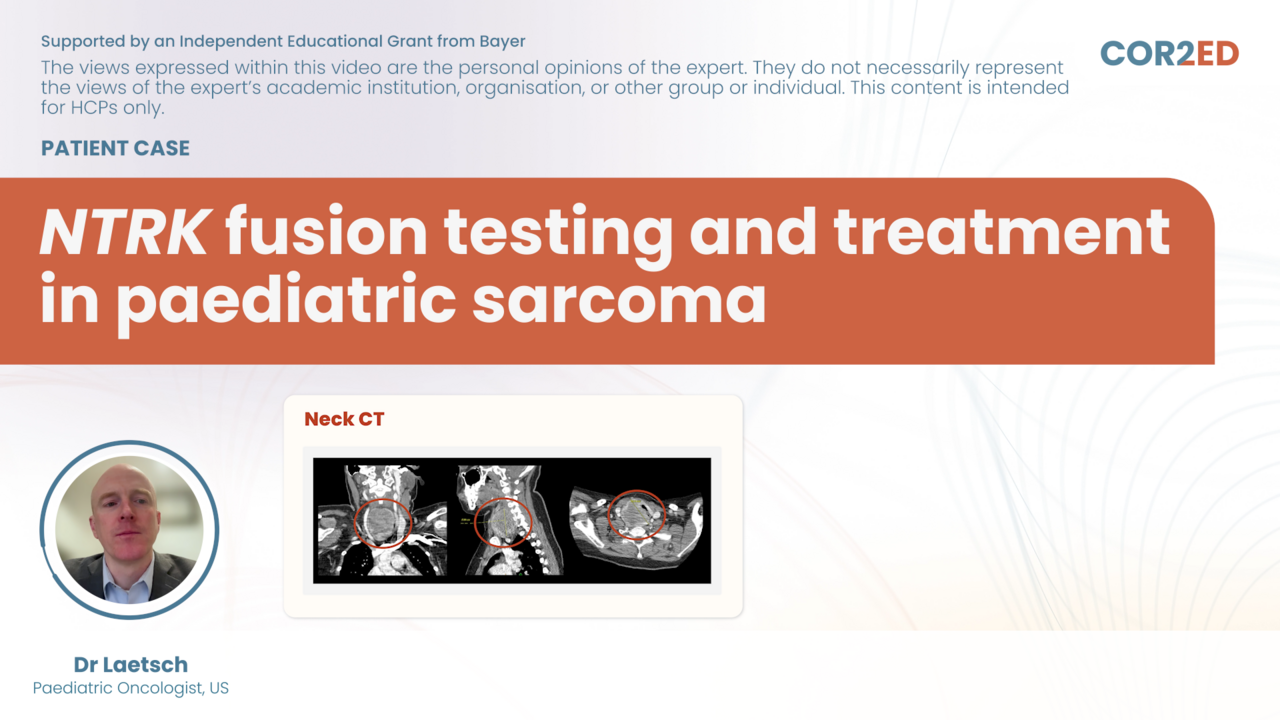
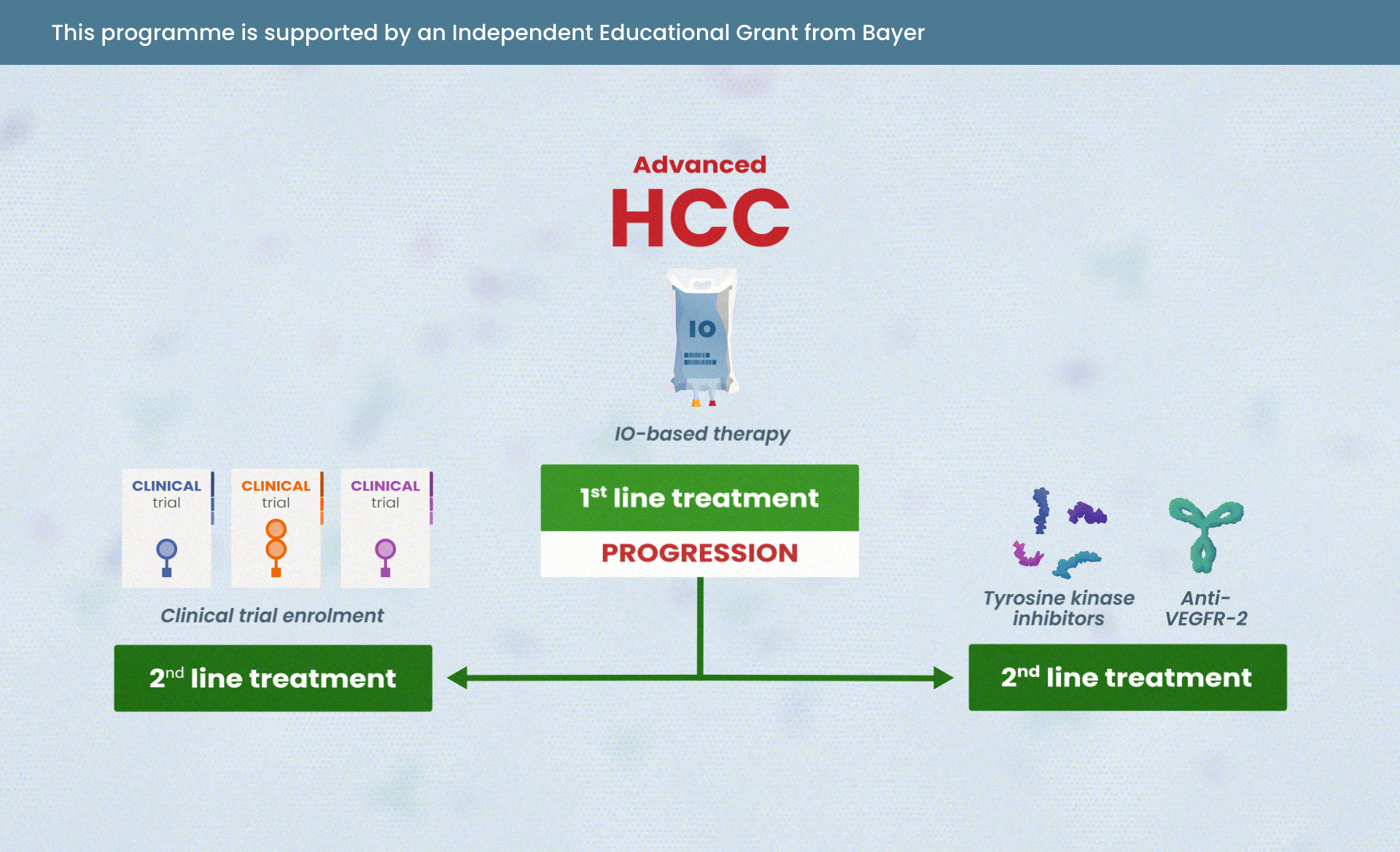
In a recent interactive webinar, renowned experts Prof. Fernando López-Ríos, Assoc. Prof. Alicia Morgans and Assoc. Prof. Herbert Loong came together to explore precision oncology in practice for lung and prostate cancer.
Watch the on-demand video to hear the medical experts discuss how to address pre-analytical phase challenges during molecular testing, and the relevant biomarkers and tests to request for lung and prostate cancer patients.
Topics of conversation included:
- An overview of common challenges related to biomarker testing across all tumour types
- Challenges and considerations of biomarker testing for lung and prostate cancer throughout the patient journey
The event concluded with speakers and participants engaging in a Q&A to address pressing questions.
Watch the video replay and download the accompanying slides for your reference, then take the e-learning to earn a CME credit (access the e-learning using the red button on the right of your screen. Or, if you're reading this on mobile, you can find the e-learning and additional resources using the red arrow on the top right of this page).
Not got time to watch the full video? Go to the video highlights: Highlights: Precision oncology in practice- testing through to treatment in lung and prostate cancer.
Clinical Takeaways
- Molecular testing with next-generation sequencing of DNA and RNA should be considered standard of care where possible using high-quality, sufficient tissue samples for accurate biomarker testing
- Pathologists should conduct ‘reflex testing’ after histologic diagnosis of lung cancer, and liquid biopsies should be utilised as a complementary testing method or when tumour tissue is not feasible
- Germline and somatic genetic testing in advanced prostate cancer may identify patients with HRR mutations and MSI-H who could be suitable for highly effective targeted agents
- Multidisciplinary team or molecular tumour board discussion can facilitate interpretation of biomarker data, leading to more accurate patient stratification, treatment selection, and clinical decision-making









 Downloadable
Downloadable  5 MIN
5 MIN
 Jun 2025
Jun 2025