Share the challenging and thought-provoking discussion at the GI CONNECT CRC Masterclass 2019. Download all presentations and take the accredited e-learning
Dr. Klempner is an Associate Professor at Massachusetts General Hospital and Harvard Medical School and leads the gastric and oesophageal programme. His clinical and translational research is centred on cancer genomics, acquired resistance to targeted therapies and the intersection of genomics and immune mediated therapies to identify novel therapeutic approaches and biomarkers in gastroesophageal cancers. He serves on the NRG non-colorectal committee, co-chairs the NCI oesophagogastric task force, and is on the NCCN guideline committees for gastric and oesophageal cancers. His work is supported by Stand Up 2 Cancer, NCI/NIH, AACR, the Degregorio Foundation, and The Gastric Cancer Foundation and Gateway for Cancer Research. He is active in gastric and oesophageal cancer outreach and patient advocacy.
Astellas, AstraZeneca, BMS, Coherus, Daiichi-Sankyo, Eli Lilly, Merck, Novartis, Nuvalent Therapuetics and Sanofi.
Dr Autumn McRee is an Associate Professor of Medicine specializing in gastrointestinal (GI) medical oncology at The University of North Carolina (UNC). She graduated from the McGovern Medical School at The University of Texas in Houston and completed her Internal Medicine residency at Vanderbilt University. During her hematology/oncology fellowship at UNC, she developed an interest in GI oncology and now as faculty, has established a career dedicated to early drug development and experimental therapeutics with an emphasis in pancreatic and hepatobiliary cancers. Dr McRee demonstrates the unique combination of clinical trial productivity and correlative science knowledge that has allowed her to seamlessly bridge the gap between scientists and clinicians with a translational research focus in tissue-based research, next generation sequencing and bioinformatics. She oversees the GI clinical trials program at UNC, serving as PI for trials both industry sponsored and investigator initiated. Dr McRee also is an active member of the Alliance for Clinical Trials in Oncology and co-chairs the Hoosier Cancer Research Network GI Clinical Trials Working Group.
Dr. Shubham Pant is a Professor in the Department of Gastrointestinal (GI) Medical Oncology with a joint appointment in the Department of Investigational Cancer Therapeutics (Phase I Center) at The University of Texas MD Anderson Cancer Center. Dr. Pant is recognised as an international expert in GI cancers with an emphasis on pancreatic and biliary cancers and Phase 1 trials.
Dr. Pant's research focuses on novel immunotherapeutic approaches and targeted therapies in GI cancers, including devising novel ways to target the KRAS mutation. He has been the lead/co-lead of several global practice-changing studies, including 'Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicenter, single-arm, phase 2b study'; 'Adagrasib in advanced solid tumors harboring a KRASG12C mutation' and 'Erdafitinib in patients with advanced solid tumors with FGFR alterations (RAGNAR): an international, single-arm, phase 2 study'; amongst others. He has over 100 peer-reviewed publications and has published in high-impact journals, including The Lancet, Lancet Oncology, and Journal of Clinical Oncology. He is frequently invited to present his research at national and international meetings. He collaborates on numerous grants, including R01 and SPORE grants funded by the National Institute of Health. Dr. Pant serves on the National Cancer Institute (NCI) Pancreas Task Force and the Pancreatic Cancer Action Network's (PanCan) Scientific and Medical Advisory Board. He also helped draft the American Society of Clinical Oncology (ASCO) Metastatic Pancreatic Cancer Guidelines, which provide evidence-based recommendations for physicians. He is also the editor of the book Pancreatic Cancer: Current Therapeutics and Future Directions (Publisher: Springer).
Alligator Bioscience, Amal Therapeutics, Arcus, AskGene Pharma, Astellas, AstraZeneca, BioNTech, Boehringer Ingelheim, BPGBio, Bristol-Myers Squibb, Elicio, Framewave, Immuneering, ImmunoMET, Ipsen, Janssen, Jazz, Lilly, Mirati Therapeutics, NGM Pharmaceuticals, Nihon Medi-Physics Co, Ltd, Novartis, Pfizer, Revolution Medicine, Theriva Biosciences, USWorldmeds, Zymeworks
Dr Joleen Hubbard is Deputy Director of Clinical Research and Academic Affairs for the Allina Health Cancer Institute in Minneapolis, Minnesota. She was previously Assistant Professor of Medical Oncology at Mayo Clinic. She completed medical school and residency at the University of Minnesota and haematology/oncology training at Mayo Clinic, Rochester, Minnesota. Dr Hubbard specialises in the treatment of gastrointestinal cancers, focusing on colorectal cancer. Dr Hubbard is the PI of several phase I clinical trials, investigating novel agents for gastrointestinal cancers. Her research interests also include geriatric oncology with a special interest in clinical and biologic markers of frailty. She serves as a member of the Cancer in the Elderly, as well as the Health Reported Outcomes and Translational Research committees for the North American Alliance of Clinical Trials in Oncology Network.
Advisory boards: Bayer, Merck, BeiGene, Incyte
Research funding to institution: Merck, Boston Biomedical, Treos Bio, Senhwa Pharmaceuticals, Bayer, Incyte, TriOncology, Seattle Genetics, Hutchison MediPharma, Pionyr Immunotherapeutics, Trovogene, G1 Therapeutics, Roche
Other programmes of interest
Innovating relapsed refractory multiple myeloma care
Unmet needs, therapy management, and real-world experience
Experts
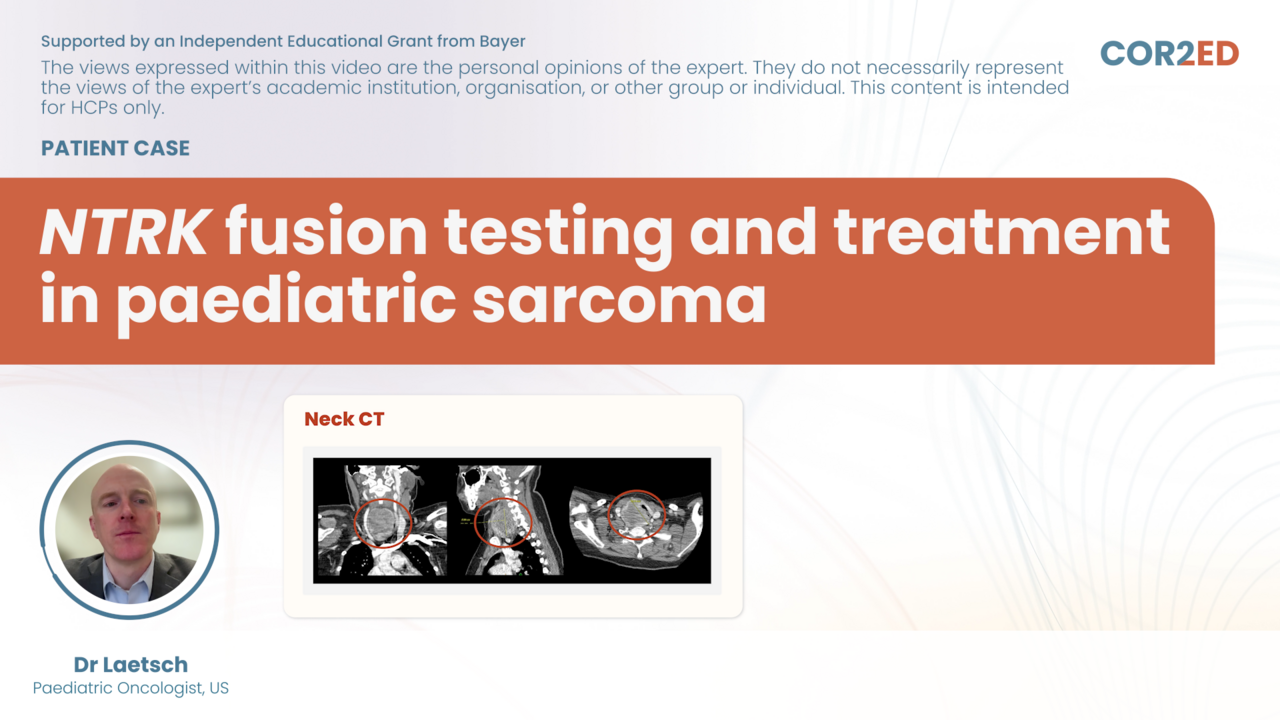
Assoc. Prof. María Victoria Mateos, Assoc. Prof. Karthik Ramasamy, Assoc. Prof. Elena ZamagniNTRK fusion testing and treatment in pediatric sarcoma
Make decisions for a young patient during his cancer journey
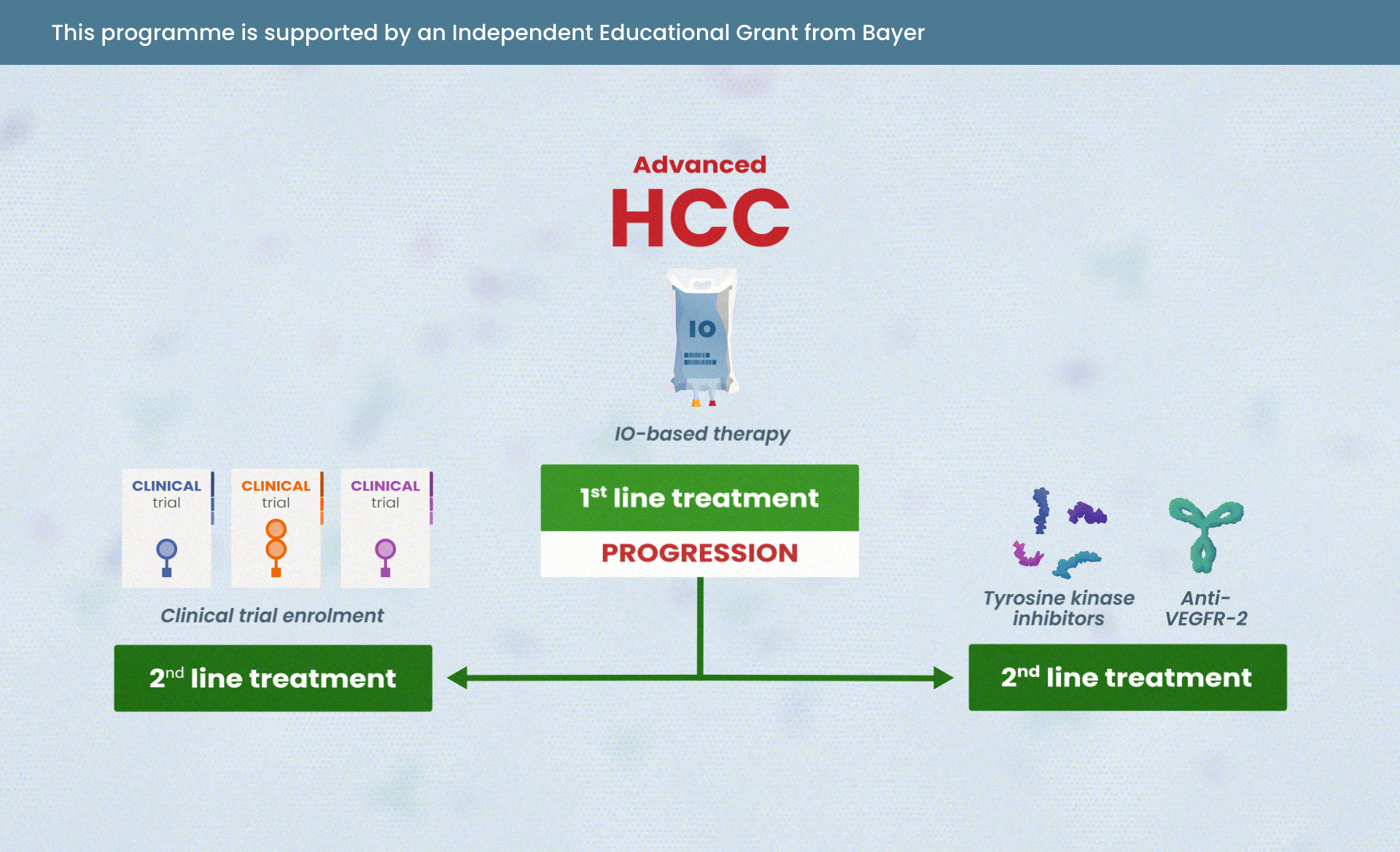
Navigating advanced HCC: treatment strategies beyond immunotherapy (IO)
Module 4: Treatment strategies for patients ineligible for IO or those with progression on IO
Later-line treatment strategies in metastatic colorectal cancer (mCRC)
Rechallenge vs switching








 Downloadable
Downloadable  5 MIN
5 MIN
 Jun 2025
Jun 2025